Mark T. Cucuzzella, Justin Tondt, Nancy E. Dockter, Laura Saslow, Thomas R. Wood
Received: 16 Sep. 2017; Accepted: 02 Nov. 2017; Published: 21 Dec. 2017
Copyright: © 2017. The Author(s). Licensee: AOSIS.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: Metabolic syndrome has become a significant problem, with the American Diabetes Association estimating the cost of diabetes and pre-diabetes in the United States alone to be $322 billion per year. Numerous clinical trials have demonstrated the efficacy of low-carbohydrate diets in reversing metabolic syndrome and its associated disorders.
Aim: This study was designed to examine how voluntary adherents to a low-carbohydrate diet rate its effectiveness and sustainability using an online survey.
Setting and methods: The 57-question survey was administered online and shared internationally via social media and ‘low-carb’ communities. Where appropriate, chi-squared tests and paired t-tests were used to analyse the responses.
Results: There were 1580 respondents. The majority of respondents had consumed less than 100 g of carbohydrates per day for over a year, typically for reasons of weight loss or disease management. There was a reported decrease in waist circumference and weight with a simultaneous decrease in hunger and increase in energy level. Of those who provided laboratory values, the majority saw improvements in their HbA1c, blood glucose measurements, and lipid panel results. There was a reduction in usage of various medications, and 25% reported medication cost savings, with average monthly savings of $288 for those respondents. In particular, the usage of pain relievers and anti-inflammatories dropped with a simultaneous decreased rating of pain and increase in mobility.
Conclusion: We conclude that low-carbohydrate diets are a sustainable method of metabolic syndrome reversal in a community setting.
Introduction
The prevalence of obesity and diabetes in the United States and the world has increased dramatically over the last 40 years with no sign of correction. These diseases remain undefeated despite billions of dollars in research and health care costs.1 Estimates from the Centers for Disease Control and Prevention (CDC) and National Health and Nutrition Examination Survey (NHANES) indicate that two-thirds of adults are overweight (body mass index (BMI) ≥25 kg/m2),2,3 with 36% being obese (BMI ≥30 kg/m2).4 The CDC’s review of 2015 data reported more than 114 million Americans have diabetes or pre-diabetes.5 While the rate of new diagnoses remains steady, the report noted that 9.4% (30.3 million of the 114 million estimated) of the US population has diabetes – an increase from 29.1 million in 2014. Countries with historically low diabetes rates are also now affected: almost half the population of China has pre-diabetes, and 10% have the diabetes.6 The medical costs of diabetes and its complications and co-morbidities are staggering. The American Diabetes Association (ADA) estimates the cost of diabetes and pre-diabetes in the United States alone to be $322 billion a year.7 Patients with diabetes account for one in three Medicare dollars spent and one in five overall health care dollars.8
At West Virginia University School of Medicine, where Dr Cucuzzella is a practising family physician and professor, outpatient and inpatient clinics are overwhelmed every day with cases of diabetes and metabolic syndrome. As of 2016, 37.7% of adults in West Virginia are obese. By best estimates, 15% have type 2 diabetes (T2D), and perhaps more than 50% have pre-diabetes.9,10 Most of these patients are completely unaware of their condition and the impacts of a poor diet, as are many of these patients’ health care providers. These patients lack the tools and support to halt or reverse their disease.
This report describes a simple approach to reversing T2D and pre-diabetes (remission is defined as normoglycaemia for 1 year without active pharmacotherapy, with the disease ‘cured’ after 5 years)11 that is showing promise in the fight against this deadly and costly disease. It is based on survey findings from more than 1500 people from West Virginia, other parts of the United States and the world who have benefited from a low-carbohydrate diet.
Metabolic syndrome as pre-diabetes
Metabolic syndrome, or ‘Syndrome X’, was first identified by endocrinologist Gerald Reaven in the 1980s, and the root cause of this condition is described as ‘insulin resistance’.12 Dr Stephen Phinney and Dr Jeff Volek explain this further as a condition of ‘carbohydrate intolerance’.13 According to recent NHANES data, almost one in three Americans now have metabolic syndrome.14 Metabolic syndrome is a sign of poor carbohydrate metabolism and hyperinsulinaemia and, if not reversed, portends an inevitable decline in overall health.15 Markers of metabolic syndrome are abdominal obesity, high blood glucose, high triglycerides (TG), low high-density lipoprotein (HDL) cholesterol and high blood pressure. Other associated conditions are increased inflammation, vascular dysfunction, non-alcoholic fatty liver disease (NAFLD), polycystic ovary syndrome, sleep apnea and some types of cancer and dementia.16,17,18,19,20,21,22,23,24 Obesity is commonly viewed as the cause of metabolic syndrome. However, while obesity is highly correlated with the condition, we (and others) suggest that it is the expansion of adipose stores beyond a person’s individual storage capacity,25,26 driven by and in conjunction with hyperinsulinaemia because of the overconsumption of processed dietary carbohydrates in the setting of a Western diet – so prevalent in modern society – that drives this disease process.26,27,28 Inflammation, especially the presence of pro-inflammatory macrophages in fat stores, such as the visceral adipose tissue, is another key marker of the developing pathology.25,29 The inflamed adipose tissue appears to lose its ability to properly respond to insulin, resulting in loss of its capacity to protectively buffer fatty acids (including sugars and starches converted to fatty acids via de novo lipogenesis to be ‘safely’ stored).25,29 This leads to reduced capacity to clear circulating glucose, as well as greater hepatic exposure to gluconeogenic precursors from rapid fatty acid turnover occurring in the insulin-resistant adipose tissue,30 both resulting in the persistently elevated blood glucose associated with metabolic syndrome.31 As a population-level intervention, restriction of carbohydrates, a key contributor to the development of metabolic syndrome (especially when processed), should therefore be considered a primary intervention for those with metabolic disease.
Type 2 diabetes reversal: Is it possible?
The prospect of reversing progressive T2D is somewhat new to both the public and health care practitioners. Many assume the diagnosis is a purely progressive disease,32,33 including an inevitable march towards worsening health and organ systems failure.34 In the lexicon of diabetes care, ‘intensive management’ has superseded ‘cure’, ‘remission’ or ‘reversal’. The ADA’s 2017 Standards of Medical Care in Diabetes states that: ‘Diabetes is a complex, chronic illness requiring continuous medical care with multifactorial risk-reduction strategies beyond glycaemic control’.35 In line with this, the ADA’s 2013 Nutrition Therapy Recommendations for the Management of Adults with Diabetes states that T2D is ‘progressive’ in nature and ‘nutrition and physical activity interventions alone (i.e. without pharmacotherapy) are generally not adequately effective in maintaining persistent glycaemic control over time for many individuals’.36
In current treatment approaches, pharmacotherapy typically is the focal point and carbohydrate restriction is de-emphasised. For instance, a recent Kaiser Permanente study involving 120 000 patients concluded that prolonged T2D remission in community settings without bariatric surgery is very rare; it occurred in only 0.007% of the study population. During the 7-year study, more study participants (1.7%) died than the percentage who experienced any level of remission, and diabetes-associated medication use, health care costs and complications increased.37 In addition, seven multinational, multicentre, randomised controlled trials aimed at achieving tight blood glucose control with medications failed to demonstrate the expected reductions in heart disease, the major killer of patients with diabetes, or in overall mortality.38,39,40,41,42,43,44 In contrast to these outcomes, strong evidence exists for a promising alternative approach.
Trials of low-carbohydrate diets: Significant findings for a new approach to type 2 diabetes
The first well-recorded version of a low-carbohydrate diet was described in 1863 by the Englishman William Banting, who restricted starchy and sugary foods to overcome obesity on the advice of his physician.45 The popularity of the diet as an effective weight loss regimen continued well into the 20th century, when it was common knowledge that ‘sugar and starches are fattening’. By mid-20th century, studies on the low-carbohydrate diet were a rarity in the medical and nutritional literature because of a growing belief that fat, and particularly saturated fat, not carbohydrate, was the more likely dietary culprit.46 Although resistance within the medical community persists,47 in recent years dietary fat has slowly been exonerated, in accordance with the best available evidence.48,49,50,51,52,53 Studies are also now emerging to show the efficacy, sustainability and other positive effects of low-carbohydrate diets, especially in those with metabolic disease.54,55,56,57,58,59,60,61
The definition of a low-carbohydrate diet varies in the literature, but most in the field agree that anything over 130 g – 150 g per day is not low carbohydrate.62 An amount of less than 50 g of carbohydrate per day is considered very low carbohydrate, which would put most adults into nutritional ketosis.63 During this state, the body relies primarily on fatty acids and ketone bodies produced from fat stores, not glucose, for energy.
Numerous randomised, controlled trials have shown that well-formulated low-carbohydrate dietary patterns are highly effective for treating obesity and improving a spectrum of risk factors typical of patients with metabolic syndrome and diabetes who previously ate a traditional diet.2,64,65,66,67,68,69,70,71 An example is a report by McKenzie et al. on the interim results of their 2-year outpatient trial on T2D patients adhering to a ketogenic diet (very low carbohydrate, usually < 50 g/day).13 Although not a randomised, controlled trial, the trial demonstrates the wide-scale applicability and efficacy of carbohydrate restriction. At 10 weeks, 91% of the 262 patients remained active in the trial and had experienced an average HbA1c reduction from 7.6% to 6.5% and an average of 7.2% body weight loss. More than half of the participants (56.8%) had reduced or eliminated one or more diabetes medications. At 6 months, 89% of the participants were retained, and weight loss averaged 12%. These findings are congruent with the physiology of insulin accelerating intracellular storage of glucose and fats, whereas reduced serum insulin from a low-carbohydrate approach allows lipolysis and gives the body access to its stored fat as an energy source.72 In contrast, intensive management of T2D with the ADA-recommended low-fat diet and standard drug regimen routinely results in minimal weight loss even when glucose control is optimised.38
In a critical review of the literature, Feinman et al. present 12 points of evidence (summarised below) to support the low-carbohydrate diet as the best first-line strategy for treating T2D and the most effective adjunct to pharmacology in type 1 diabetes (T1D)55:
- Dietary carbohydrate restriction has the greatest effect on decreasing abnormally high blood glucose levels, the most salient feature of diabetes.
- Increased caloric intake, the driver of the obesity and T2D epidemics, has been due almost entirely to increased carbohydrate consumption.
- Weight loss is not required to reap the benefits of a low-carbohydrate diet, which include reversal of T2D.
- For weight loss, no other dietary intervention has proven to be better than carbohydrate restriction.
- For people with T2D, the low-carbohydrate diet is as effective as other dietary interventions and often significantly better.
- Replacement of carbohydrate with protein generally improves glycaemic control.
- Total fat and saturated fat intake do not correlate with risk of cardiovascular disease.
- Plasma-saturated fatty acid levels are affected more by eating carbohydrates than eating fats.
- The best predictor of microvascular, and to a lesser extent macrovascular, complications in patients with T2D is glycaemic control (as measured by HbA1c).
- Dietary carbohydrate restriction is the most effective method (other than starvation) for reducing serum TG and increasing high-density lipoproteins.
- Low-carbohydrate diets reduce and frequently eliminate medications for T2D patients and usually result in lower insulin doses for patients with T1D.
- Intensive glucose lowering by carbohydrate restriction has none of the side effects associated with intensive pharmacologic therapies.
Despite a growing body of evidence for low-carbohydrate diets’ superior results in the management of obesity, cardiovascular disease risk, metabolic syndrome and T2D, objections by the medical and public health communities persist.73 This is frequently based on flawed or outdated evidence.74 For example, misunderstanding about the body’s need for glucose, in particular by the central nervous system, continues despite basic scientific knowledge to the contrary.5 In concert, the ADA does not recommend a low-carbohydrate diet for diabetes management. While recognising that ‘carbohydrate intake has a direct effect on postprandial glucose levels’ and ‘total amount of carbohydrate eaten is the primary predictor of glycaemic response’, the ADA advises that ‘a variety of eating patterns (combinations of different foods or food groups) are acceptable for the management of diabetes’ and recommends adjusting carbohydrate intake to mealtime insulin dosing.36 The ADA has refrained from making a robust recommendation for carbohydrate restriction. It posits that ‘evidence is inconclusive for an ideal amount of carbohydrate intake for people with diabetes’.36
Aim and objectives
Although a reduced-carbohydrate approach may theoretically improve health, if it is too difficult to follow long term or if it brings with it unwanted effects, the idea that people should reduce their carbohydrates is at best irrelevant. Our hypothesis is that if one adheres to a well-formulated low-carbohydrate diet, the success and health benefits can be maintained over years and even for a lifetime. To probe the real-life utility of such an approach, previous examinations of people following some kind of carbohydrate restriction have been conducted. For example, Tim Noakes, a South African physician and low-carbohydrate diet researcher, published an analysis of more than 100 communications he had received from people following some kind of carbohydrate restriction. The participants reported reduced weight, hunger, irritable bowel syndrome symptoms, hypertension and medication needs, as well as improved glycaemic control and exercise capacity.75 A survey of more than 2000 members of an online, low-carbohydrate support group found positive health outcomes for participants, such as decreased body weight and an improved lipid profile.76 A survey of more than 2500 people following a low-carbohydrate diet of some type (or a paleo diet, which is often a reduced-carbohydrate diet) found a variety of positive health benefits such as reduced weight, hunger, irritable bowel syndrome symptoms, joint pain, arthritis pain, brain fog and acne, as well as improved glycaemic control, psychological well-being, athletic performance and energy.77 About 100 people from the National Weight Control Registry, which tracks people who have long-term weight loss success, reported using a low-carbohydrate diet approach and reported long-term weight loss with reduced hunger.78
The purpose of this research was to gain a detailed, updated understanding of adults who were already voluntarily following a low-carbohydrate diet. Because long-term dietary trials are complex and costly, we were especially interested in the experience of long-term diet adherents. Such research is hypothesis-generating and enables researchers to explore the actual lived experience of people following such a dietary approach.
Methods
Study design
The Low Carb Lifestyle Survey was a quality improvement project for the American Board of Family Physicians (ABFM), developed by Dr Mark Cucuzzella and with survey design assistance from collaborators in the United Kingdom (diabetes.co.uk).
Setting, study population and sampling strategy
The survey was shared with the American and international ‘low-carb’ community via social media and through international health care professionals who support therapeutic uses of low-carbohydrate diets. As Dr Cucuzzella has been recommending a low-carbohydrate diet as an option to his patients with T2D for 6 years, the survey was also shared with individual patients he sees and members of his local community.
Data collection
The 57-question survey was administered on Survey Monkey (an online survey company); the survey was open from December 2016 to June 2017. The survey included closed-ended, multiple-choice and open-ended questions. Responses to the survey were kept confidential. The full 57-question survey can be viewed in supplemental materials (see Appendix 1).
Data analysis
Where appropriate, we used chi-squared tests to compare proportions and paired t-tests to compare means and standard deviation changes within the same groups. Statistical analyses were performed using IBM SPSS software Version 22 (SPSS Inc., Chicago, IL) and GraphPad Prism version 7 (GraphPad Software, La Jolla, CA).
Ethical considerations
The American Board of Family Medicine (ABFM) peer-reviewed and approved the project before release. After results were collected, the ABFM again reviewed the project and determined that it met standards for a Quality Improvement (QI) project. A QI project relevant to the quality and standards of the ABFM is mandatory for maintenance of certification as a Board Certified Family Physician.
Results
Survey respondent characteristics
The survey was completed by 1580 respondents. Respondents were required to answer all questions, with the exception of the open-ended items. The majority of respondents resided in the United States, with the rest residing elsewhere (Table 1). Most of the respondents were female, white, middle-aged and working full time. Of the survey respondents, 97% said that they considered themselves to be adherent to a low-carbohydrate diet. More than half of the respondents said that they had been on a low-carbohydrate diet for at least 1 year. The most commonly reported way respondents had learned about the low-carbohydrate diet was through the Internet. Respondents were allowed to choose more than one reason for starting a low-carbohydrate diet. Three out of four respondents said they started the diet for weight loss, but chronic disease improvement and a desire for more energy were also common responses.
TABLE 1: Survey respondent characteristics.
Almost half (49%) of the respondents reported a very restricted average carbohydrate intake of less than 30 g per day. Almost one-third (32%) reported an average carbohydrate intake of 30 g – 50 g per day, 16% reported 50 g – 100 g per day and the remaining 3% reported more than 100 g per day. Average carbohydrate intake was inversely associated with amount of time spent on the diet; that is, of those adherents for less than 6 months, 65% reported an average daily carbohydrate intake of less than 30 g. For participants adherent from 6 months to 2 years, daily consumption of less than 30 g daily still comprised the majority response, but had declined with time. By 2 years, those consuming less than 30 g daily and those consuming 30 g – 50 g daily were even at 38% each. This is consistent with one approach to carbohydrate restriction, starting with a very low-carbohydrate intake and then gradually increasing the carbohydrate level as time goes on.79 The amount of weekly physical activity reported was an average of 164 min, with 18% reporting no regular physical activity.
Weight loss and lab test values
The large majority of respondents reported weight loss and waist circumference reduction with a low-carbohydrate diet (Table 2). Three out of four reported weight loss of 10 pounds or more, with more than one-third of respondents reporting a weight loss of more than 30 pounds. Similarly, 81% respondents reported some reduction in waist size, with 17% reporting a reduction of 5 inches or more. Decreases in weight and waist circumference correlated inversely with daily carbohydrate intake, with a greater proportion of participants (those consuming less than 30 g of carbohydrate per day) reporting more than 3 inches lost from the waist or more than 20 pounds total weight loss (Figure 1).

TABLE 2: Weight and waist circumference change with low-carbohydrate diet.
Long-term weight loss and waist circumference reduction
Among respondents who knew their weight and waist circumference before and after diet initiation, at 6–12 months on the diet (Figure 2), 65% reported a weight loss of 20 pounds or more and 52% reported a waist circumference reduction of 3 inches or more. We found similar results for people who reported following a reduced carbohydrate diet for even longer. For example, for those on the diet 2 years or more, 59% reported a weight loss of 20 pounds or more and 46% reported a waist circumference reduction of 3 inches or more. Providing laboratory values was optional in the survey. Lab test values of respondents who knew their values before diet initiation and after are shown in Tables 3 and 4. Improvements were reported for fasting blood glucose, postprandial blood glucose and triglyceride/HDL (TG/HDL) ratio (Table 3).
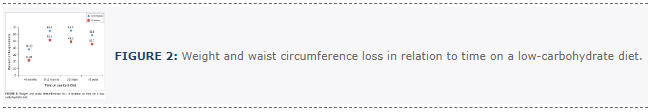

TABLE 3: Lab test values before and after low-carbohydrate diet initiation.
TABLE 4: Proportion of participants with HbA1c levels in the normal, pre-diabetes or type 2 diabetes ranges before and after initiation of a low-carbohydrate diet (N = 495).
Glycaemic control
We also examined the proportion of participants reporting HbA1c levels in the normal range or in the ranges diagnostic of pre-diabetes or T2D (Table 4). We noticed large improvements. For example, the proportion of participants reporting an HbA1c below 5.5 increased almost fourfold (17% to 65%). Almost half (49%) reported an HbA1c of 6.5 or more before diet initiation. After initiation, fewer than one in 10 (7%) reported an HbA1c that high. Absolute increase in participants reporting a normal HbA1c (< 5.5; 48.7% increase) and absolute decrease in participants reporting a diabetic HbA1c (≥ 6.5; 41.4%) were both greatest in the group eating less than 30 g of carbohydrate per day, with smaller absolute improvements with increasing carbohydrate intake (Table 5).
| TABLE 5: HbA1c values stratified by carbohydrate intake. |
Medication use
Respondents reported declines in use of medications (e.g., antidepressant, anti-anxiety, sleep aids, pain relief, anti-inflammatory and phosphodiesterase type 5 inhibitors for erectile dysfunction) after starting the diet (Table 6). The use of all types of medications (except for erectile dysfunction) reduced by more than 50%.
| TABLE 6: Medication use before and after low-carbohydrate diet initiation. |
Small minorities of respondents reported current use of medications for diabetes, high blood pressure and hyperlipidaemia, with some reporting having reduced or completely discontinued such medications after initiating the diet (Table 7). While more than half of the respondents said that medication costs were not relevant for them, (e.g. NHS on no medication costs or not on medication) 25% reported a savings, with the average reported savings in medications cost being $288 per month.
| TABLE 7: Medication use. |
Energy, mobility, pain, physical and emotional well-being, and between-meal state
Respondents reported improvements in energy level (see Figure 3), ability to perform usual activities (such as housework or leisure activities), pain and emotional health after starting a low-carbohydrate diet (Table 8). For example, before initiation, 59% of respondents reported low energy levels and only 4% reported high energy levels, compared to 3% and 51%, respectively, after initiation. The numbers of respondents reporting moderate to extreme problems with mobility, pain or symptoms of anxiety or depression declined after diet initiation (Table 8). Respondents reported improvement in a variety of aspects of physical and psychological well-being after starting a low-carbohydrate diet. For example, at least eight out of 10 respondents reported improvement in outlook, happiness and self-esteem, and six out of 10 reported improved confidence in controlling blood sugar (Table 9). Similarly, before initiating a low-carbohydrate diet, the great majority of respondents reported negative between-meal experiences, such as intense hunger, tiredness and difficulty concentrating (Table 10). Fewer than one in five respondents reported having such experiences after diet initiation.


TABLE 8: Energy and activity, physical and emotional well-being before and after low-carbohydrate diet initiation.
TABLE 9: Changes in physical and psychological well-being after starting low-carbohydrate diet.
TABLE 10: Between-meal experiences.
Other health changes, challenges and knowledge gained
Respondents were invited to answer three open-ended questions about their experiences on a low-carbohydrate diet. Common responses are summarised in Table 11. Commonly reported health changes were improvements on a wide spectrum: breathing and allergies, markers of NAFLD, libido and reproductive health, mental clarity and digestion. Commonly reported challenges related to a low-carbohydrate diet included negative reactions from family, friends and health professionals; dining out, special occasions and travelling; flu-like symptoms associated with transitioning to a low-carbohydrate diet; and desires for certain foods. Common reports of the important learning or information gained from being on a low-carbohydrate diet pertained to insulin resistance and metabolic syndrome; the difference between nutritional ketosis and ketoacidosis; and awareness about how different foods affect satiety, weight gain and how one feels.
TABLE 11: Commonly reported free-response answers.
Discussion
To date, few studies have been published on the efficacy of low-carbohydrate diets for weight loss or reversal of metabolic syndrome and T2D beyond 2 years in duration.80 Our survey offers a unique perspective on this issue. It garnered a large, international cohort of individuals, with more than 500 of them being adherent to a low-carbohydrate diet for more than 2 years. Because respondents were overwhelmingly people succeeding on the diet, the data give an undiluted view of what is possible when a low-carbohydrate approach is successful. The reversals in lab values, body weight and waist circumference that were reported in the survey are striking. In addition, reductions in medication use, pain and mental health concerns along with improved energy and mobility after diet initiation were commonly reported. As would be expected with such improvements in physical health, the cohort reported overwhelmingly that their physical and psychological well-being also improved after starting a low-carbohydrate diet.
Discussion of key findings
An important marker of metabolic health and adverse long-term outcomes is a large waist circumference and visceral fat.81,82 Although we had to rely on participants’ self-report of pounds and inches gained or lost, in light of the fact that sustained weight loss is universally difficult, our dramatic findings deserve consideration. A weight loss of 20 pounds or more was reported by 65.1% of those on the diet for 6–12 months and by 58.8% of those on the diet for 2 years or more (Figure 2).
Although fewer than half (44.1%) of respondents reported that their reason for following a low-carbohydrate diet was to improve a chronic condition, we collected robust data for diabetes and glucose control. Of the entire cohort, 495 knew their HbA1c before diet initiation and 413 knew their value post-diet initiation. By definition, people with T2D have an HbA1c of ≥ 6.5, and pre-diabetes is defined by an HbA1c of 5.7–6.4. Many studies suggest that an HbA1c around 5.0–5.5 is likely to be the ideal.83,84,85,86 Of those reporting their after-initiation values, the proportion with an HbA1c < 6.5 nearly doubled, and nearly four times as many reported an HbA1c of < 5.5. Most importantly, those with an HbA1c in the diabetic range (≥ 6.5) decreased from 48.5% to 7.1%.
Active management of diabetes requires measuring fasting and post-meal blood glucose levels. Average pre-diet values (143 mg/dL fasting and 175 mg/dL post-meal) reflected poor glucose tolerance (Table 3). At the time of the survey, the average blood glucose values were 99 mg/dL for fasting and 107 mg/dL for post-meal, which is a significant improvement.
It is clear in the literature that the most powerful predictor in a basic lipid panel of insulin resistance and cardiovascular health is the ratio of TG and HDL-cholesterol.87,88,89,90 As low-density lipoprotein (LDL) cholesterol (which is calculated as the measurement of cholesterol mass within LDL particles) is made up of small (atherogenic) and large (healthy) particles, there is controversy about total LDL role as a predictor of cardiovascular events.74 A low TG/HDL ratio reflects ‘pattern A’ (large LDL particles) and a high TG/HDL ratio reflects ‘pattern B’ (small LDL particles), the latter being more closely associated with both carbohydrate (particularly refined) intake and cardiovascular disease risk.90,91 Because of its association with a less atherogenic lipid profile, a TG/HDL ratio close to 1.0 is regarded as predictive of low cardiovascular risk.92 A higher TG/HDL ratio is also associated with hyperinsulinaemia,93 which is an independent risk factor for cardiovascular disease.94 One concern about a low-carbohydrate diet is the typical slight rise in LDL cholesterol, although no study has shown its adverse outcome. Dietary interventions that lower LDL have also failed to show reductions in disease risk.95,96 In fact, evidence has shown overall cardiovascular risk to improve on a well-formulated low-carbohydrate diet.97 Higher LDL-C has been associated with longevity in one review of 68 000 patients.98 Among study respondents who knew their values, after starting a low-carbohydrate diet, average HDL increased and average TG decreased. Most significantly, the pre-diet TG/HDL ratio was 3.5. After diet initiation, the average TG/HDL ratio was 1.4, which reflects a much lower cardiovascular risk.
Our survey showed marked reductions in medication use and costs, which is an important finding not only for consumers but also for insurance companies and taxpayers. While more than half of the respondents said drug costs were not relevant for them, a quarter of respondents were able to reduce their individual costs, and in those with a specific amount cited, the average reduction was $288 a month.
Decreased strength and difficulty in mobility are associated with poor health outcomes and increased mortality.99,100,101 The ability to rise from the floor and have a strong walking speed is associated with healthy ageing.102 The number of survey respondents reporting moderate or severe mobility problems or being unable to walk declined from 278 to 49 individuals after diet initiation, and the number reporting moderate to severe pain declined dramatically after diet initiation, from 674 to 78. It is reasonable to conclude that the reported improvements in mobility were at least in part associated with reductions in pain. Chronic pain is a major reason why patients seek primary care, and our current approach to pain management has resulted in unintended consequences, contributing to the current opioid crisis.103 Safe and natural methods for pain management need to be explored, and we are just beginning to learn the potential of nutritional therapy. This finding suggests further work needs to be conducted on dietary interventions for pain.
Similarly, anxiety and depression are common primary care symptoms.104 Pharmaceutical management of these conditions has shown marginal benefit (confounded by significant misreporting of trials in the literature),105,106 especially in those with mild or moderate symptoms where dietary or lifestyle interventions are likely to have a greater impact.107,108 Antidepressant medications also carry significant side effects, especially in elderly patients already under a burden of iatrogenic polypharmacy.109 Our survey respondents reported marked improvements in symptoms of anxiety and depression after starting a low-carbohydrate diet. These findings support low-carbohydrate nutrition as an alternative therapeutic approach for some mental health conditions and warrant continued research.110 How one feels between meals in mood and cravings is also critical to sustain any nutritional lifestyle plan. One well-known claim of low-carbohydrate diets is that one can lose weight and not be hungry.111,112,113 Our survey respondents, more than one-third of whom had been on a low-carbohydrate diet over 2 years, strongly affirmed this. Table 10 shows the dramatic differences before and after low-carbohydrate diet initiation in self-reported symptoms of hunger, fatigue, poor concentration, mood swings, blood sugar swings, irritability and anxiety. Furthermore, participants also reported an increase in energy levels despite the lower caloric intake that naturally occurs with a low-carbohydrate diet.114
In this sample of successful, mostly long-term low-carbohydrate diet followers, the diet led to improvements in physical and psychological well-being for most of our study respondents. Feelings of emotional well-being are hypothesised to be important components for sustainable lifestyle change.115 These overwhelmingly positive changes suggest that a low-carbohydrate diet may be especially sustainable. In addition, 18% of participants reported no regular physical activity, supporting the idea that people do not need to exercise excessively to lose fat and improve metabolism.
Moreover, participants reported improvements in a variety of health conditions after initiating a low-carbohydrate diet, some of which have been studied before, at least preliminarily (improvements in symptoms related to migraines,116 irritable bowel syndrome,117 heartburn,118 polycystic ovary syndrome,119 NAFLD120 and pain121), and some of which have not been studied well or much at all (e.g., libido, immune function and allergies). More research is needed on these topics.
Limitations
This study was not without limitations. This was a retrospective study assessing a host of biomedical indicators reliant on self-reported data subject to recall bias. Although we believe that the questions we used are face valid, we did not use validated self-report scales. Therefore, some answers may not be as reliable and accurate as they would have been if we had used validated instruments. A detailed survey like this also lends itself to those who are highly engaged in the topic. Like any survey, the respondents may not be representative of all patients. Furthermore, in our appeal for participants, we did not differentiate between people who are followers of one of the many types of carbohydrate-restricted approaches such as low-carbohydrate, high-fat and low-carbohydrate, high-protein (although most of the practitioners who supported the survey favoured a low-carbohydrate, high-fat approach). As to the small sample of minorities, we feel this reflects, at least in part, the bias inherent in snowballing sampling. Many of the initial contacts when the survey was launched were not minority (non-white people comprise about 6% of the population of the state of West Virginia). As the survey was largely shared through social networks, it could be that race played a role in who was invited to take part.
Furthermore, this study lacked the rigour of a randomised, controlled trial. However, our goal was not to compare the results of a low-carbohydrate diet with some other dietary approach. Instead, we were interested in what people who were voluntarily choosing to follow some kind of carbohydrate-reduced nutritional approach would report about their lived experience.
Implications
The results of our survey support pursuit of other pertinent questions around myriad aspects of low-carbohydrate diets: long-term health effects, factors that facilitate success and overcoming of barriers, age-related differences in response to the diet and parameters of the optimal low-carbohydrate diet for overall health and well-being.
Conclusion
Currently recommended drug treatments, nutritional guidelines and behavioural interventions have had limited to no success in halting the obesity and diabetes epidemics. This has resulted in exorbitant health care costs122 and indirect costs from lower quality of life123 and lost productivity124 for a growing proportion of the population. The premise of this research project is that excess carbohydrate intake in the Western diet is a major driver of the obesity and diabetes epidemics, and that restricting dietary carbohydrate to a level that permits utilisation of fat as the primary fuel will yield substantial weight loss and improvement in diabetes and metabolic syndrome, other health conditions and overall quality of life. Moreover, we show that for many, a low-carbohydrate diet is satisfying and sustainable.
Acknowledgements
Competing interests
The authors declare that they have no financial or personal relationships which may have inappropriately influenced them in writing this article.
Authors’ contributions
M.T.C. was responsible for project conception, design and implementation. J.T. assisted with project implementation, performed calculations and created figures and graphs. M.T.C. and J.T. wrote the initial draft. N.E.D. made conceptual and editorial contributions. L.S. was responsible for the aims section and made conceptual and editorial contributions. T.R.W. made conceptual and editorial contributions and reviewed calculations as well as directed the reference section.
References
- CDC. National diabetes statistics report, 2017. Atlanta GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
- Brinkworth GD, Noakes M, Buckley JD, Keogh JB, Clifton PM. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am J Clin Nutr. 2009;90:23–32. https://doi.org/10.3945/ajcn.2008.27326
- Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS data brief, no 219. Hyattsville, MD: National Center for Health Statistics; 2015.
- Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. https://doi.org/10.1001/jama.2016.6458
- Manninen AH. Metabolic effects of the very-low-carbohydrate diets: Misunderstood ‘villains’ of human metabolism. J Int Soc Sports Nutr. 2004;1:7–11. https://doi.org/10.1186/1550-2783-1-2-7
- Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317:2515–2523. https://doi.org/10.1001/jama.2017.7596
- Dall TM, Yang W, Halder P, et al. The economic burden of elevated blood glucose levels in 2012: Diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care. 2014;37:3172–3179. https://doi.org/10.2337/dc14-1036
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046. https://doi.org/10.2337/dc12-2625
- The state of obesity – West Virginia [homepage on the Internet]. 2016 [cited 2017 Sept 14]. Available from: https://stateofobesity.org/states/wv
- Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314:1021–109. https://doi.org/10.1001/jama.2015.10029
- Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care. 2009;32:2133–2135. https://doi.org/10.2337/dc09-9036
- Olefsky J, Farquhar JW, Reaven G. Relationship between fasting plasma insulin level and resistance to insulin-mediated glucose uptake in normal and diabetic subjects. Diabetes. 1973;22:507–513. https://doi.org/10.2337/diab.22.7.507
- McKenzie AL, Hallberg SJ, Creighton BC, et al. A novel intervention including individualized nutritional recommendations reduces hemoglobin A1c level, medication use, and weight in type 2 diabetes. JMIR Diabetes. 2017;2:e5. https://doi.org/10.2196/diabetes.6981
- Mozumdar A, Liguori G. Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999–2006. Diabetes Care. 2011;34:216–219. https://doi.org/10.2337/dc10-0879
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death. J Am Coll Cardiol. 2007;49:403–414. https://doi.org/10.1016/j.jacc.2006.09.032
- Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. 2012;32:1754–1759. https://doi.org/10.1161/ATVBAHA.111.241885
- Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–150. https://doi.org/10.1016/j.diabres.2014.04.006
- Tziomalos K, Athyros VG, Karagiannis A, Mikhailidis DP. Endothelial dysfunction in metabolic syndrome: Prevalence, pathogenesis and management. Nutr Metab Cardiovasc Dis. 2010;20:140–146. https://doi.org/10.1016/j.numecd.2009.08.006
- Williams T. Metabolic syndrome: Nonalcoholic fatty liver disease. FP Essent. 2015;435:24–29.
- Essah PA, Wickham EP, Nestler JE. The metabolic syndrome in polycystic ovary syndrome. Clin Obstet Gynecol. 2007;50:205–225. https://doi.org/10.1097/GRF.0b013e31802f3547
- Calvin AD, Albuquerque FN, Lopez-Jimenez F, Somers VK. Obstructive sleep apnea, inflammation, and the metabolic syndrome. Metab Syndr Relat Disord. 2009;7:271–277. https://doi.org/10.1089/met.2008.0093
- Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: A systematic review and meta-analysis. Diabetes Care. 2012;35:2402–2411. https://doi.org/10.2337/dc12-0336
- Ng TP, Feng L, Nyunt MS, et al. Metabolic syndrome and the risk of mild cognitive impairment and progression to dementia: Follow-up of the Singapore longitudinal ageing study cohort. JAMA Neurol. 2016;73:456–463. https://doi.org/10.1001/jamaneurol.2015.4899
- Solfrizzi V, Scafato E, Capurso C, et al. Metabolic syndrome, mild cognitive impairment, and progression to dementia. The Italian Longitudinal Study on Aging. Neurobiol Aging. 2011;32:1932–1941. https://doi.org/10.1016/j.neurobiolaging.2009.12.012
- Rosen Evan D, Spiegelman Bruce M. What we talk about when we talk about fat. Cell. 2014;156:20–44. https://doi.org/10.1016/j.cell.2013.12.012
- Kim JI, Huh JY, Sohn JH, et al. Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Mol Cell Biol. 2015;35:1686–1699. https://doi.org/10.1128/MCB.01321-14
- Crofts CAP, Zinn C, Wheldon M, Schofield G. Hyperinsulinemia: A unifying theory of chronic disease? Diabesity. 2015;1(4):34–43. https://doi.org/10.15562/diabesity.2015.19
- Williams KJ, Wu X. Imbalanced insulin action in chronic over nutrition: Clinical harm, molecular mechanisms, and a way forward. Atherosclerosis. 2016;247:225–282. https://doi.org/10.1016/j.atherosclerosis.2016.02.004
- Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:139239. https://doi.org/10.1155/2013/139239
- Perry Rachel J, Camporez J-PG, Kursawe R, et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160:745–758. https://doi.org/10.1016/j.cell.2015.01.012
- Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992;90:1323–1327. https://doi.org/10.1172/JCI115997
- U.K. Prospective Diabetes Study 16: Overview of 6 years’ therapy of type II diabetes: A progressive disease. Diabetes. 1995;44:1249–1258. https://doi.org/10.2337/diab.44.11.1249
- American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care. 2015;38:S8–S16. https://doi.org/10.2337/dc15-S005
- Soumya D, Srilatha B. Late stage complications of diabetes and insulin resistance. J Diabetes Metab. 2011;2:167. https://doi.org/10.4172/2155-6156.1000167
- Introduction. Diabetes Care. 2017;40:S1–S2. https://doi.org/10.2337/dc17-S001
- Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821–3842. https://doi.org/10.2337/dc13-2042
- Karter AJ, Nundy S, Parker MM, Moffet HH, Huang ES. Incidence of remission in adults with type 2 diabetes: The diabetes & aging study. Diabetes Care. 2014;37:3188–3195. https://doi.org/10.2337/dc14-0874
- Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. https://doi.org/10.1056/NEJMoa0802743
- Zoungas S, Chalmers J, Neal B, et al. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371:1392–1406. https://doi.org/10.1056/NEJMoa1407963
- Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197–2206. https://doi.org/10.1056/NEJMoa1414266
- Gerstein HC, Bosch J, Dagenais GR, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–328. https://doi.org/10.1056/NEJMoa1203858
- Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242. https://doi.org/10.1056/NEJMoa1501352
- Hirshberg B, Katz A. Insights from cardiovascular outcome trials with novel antidiabetes agents: What have we learned? An industry perspective. Curr Diab Rep. 2015;15:87. https://doi.org/10.1007/s11892-015-0663-9
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. https://doi.org/10.1007/s11892-015-0663-9
- Banting W. Letter on corpulence. Washington, DC: U.S. National Library of Medicine; 1984.
- Taubes G. Good calories, bad calories. New York: Anchor Bookis, Random House, Inc.; 2008.
- Sacks FM, Lichtenstein AH, Wu JHY, et al. Dietary fats and cardiovascular disease: A presidential advisory from the American Heart Association. Circulation. 2017;136(10):e195. https://doi.org/10.1161/CIR.0000000000000510
- Dehghan M, Mente A, Zhang X, et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet. 2017;390:2050–2062. https://doi.org/10.1016/S0140-6736(17)32252-3
- Harcombe Z, Baker JS, Cooper SM, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: A systematic review and meta-analysis. Open Heart. 2015;2:e000196. https://doi.org/10.1136/openhrt-2014-000196
- Chowdhury R, Warnakula S, Kunutsor S, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann Intern Med. 2014;160:398–406. https://doi.org/10.7326/M13-1788
- Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr. 2010;91:535–546. https://doi.org/10.3945/ajcn.2009.27725
- De Souza RJ, Mente A, Maroleanu A, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ. 2015;351:h3978. https://doi.org/10.1136/bmj.h3978
- Hamley S. The effect of replacing saturated fat with mostly n-6 polyunsaturated fat on coronary heart disease: A meta-analysis of randomised controlled trials. Nutr J. 2017;16:30. https://doi.org/10.1186/s12937-017-0254-5
- Tobias DK, Chen M, Manson JE, Ludwig DS, Willett W, Hu FB. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:968–979. https://doi.org/10.1016/S2213-8587(15)00367-8
- Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition. 2015;31:1–13. https://doi.org/10.1016/j.nut.2014.06.011
- Volek JS, Phinney SD, Forsythe CE, et al. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids. 2009;44:297–309. https://doi.org/10.1007/s11745-008-3274-2
- Hjorth MF, Ritz C, Blaak EE, et al. Pretreatment fasting plasma glucose and insulin modify dietary weight loss success: Results from 3 randomized clinical trials. Am J Clin Nutr. 2017;106:499–505. https://doi.org/10.3945/ajcn.117.155200
- Saslow LR, Mason AE, Kim S, et al. An online intervention comparing a very low-carbohydrate ketogenic diet and lifestyle recommendations versus a plate method diet in overweight individuals with type 2 diabetes: A randomized controlled trial. J Med Internet Res. 2017;19:e36. https://doi.org/10.2196/jmir.5806
- Hu T, Yao L, Reynolds K, et al. The effects of a low-carbohydrate diet vs. a low-fat diet on novel cardiovascular risk factors: A randomized controlled trial. Nutrients. 2015;7:7978–7994. https://doi.org/10.3390/nu7095377
- Perez-Guisado J, Munoz-Serrano A, Alonso-Moraga A. Spanish ketogenic Mediterranean diet: A healthy cardiovascular diet for weight loss. Nutr J. 2008;7:30. https://doi.org/10.1186/1475-2891-7-30
- Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: Comparison of low-carbohydrate and low-fat diets. A meta-analysis. PLoS One. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
- Westman EC, Feinman RD, Mavropoulos JC, et al. Low-carbohydrate nutrition and metabolism. Am J Clin Nutr. 2007;86:276–284.
- Volek JS, Feinman RD. Carbohydrate restriction improves the features of metabolic syndrome. Metabolic syndrome may be defined by the response to carbohydrate restriction. Nutr Metab. 2005;2:31. https://doi.org/10.1186/1743-7075-2-31
- Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: The A to Z weight loss study: A randomized trial. JAMA. 2007;297:969–977. https://doi.org/10.1001/jama.297.9.969
- Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–241. https://doi.org/10.1056/NEJMoa0708681
- Guldbrand H, Dizdar B, Bunjaku B, et al. In type 2 diabetes, randomisation to advice to follow a low-carbohydrate diet transiently improves glycaemic control compared with advice to follow a low-fat diet producing a similar weight loss. Diabetologia. 2012;55:2118–2127. https://doi.org/10.1007/s00125-012-2567-4
- Bazzano LA, Hu T, Reynolds K, et al. Effects of low-carbohydrate and low-fat diets: A randomized trial. Ann Intern Med. 2014;161:309–318. https://doi.org/10.7326/M14-0180
- Foster GD, Wyatt HR, Hill JO, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: A randomized trial. Ann Intern Med. 2010;153:147–157. https://doi.org/10.7326/0003-4819-153-3-201008030-00005
- Stern L, Iqbal N, Seshadri P, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: One-year follow-up of a randomized trial. Ann Intern Med. 2004;140:778–785. https://doi.org/10.7326/0003-4819-140-10-200405180-00007
- Tay J, Luscombe-Marsh ND, Thompson CH, et al. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: A randomized trial. Am J Clin Nutr. 2015;102:780–790. https://doi.org/10.3945/ajcn.115.112581
- Sondike SB, Copperman N, Jacobson MS. Effects of a low-carbohydrate diet on weight loss and cardiovascular risk factor in overweight adolescents. J Pediatr. 2003;142:253–258. https://doi.org/10.1067/mpd.2003.4
- Kersten S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2001;2:282–286. https://doi.org/10.1093/embo-reports/kve071
- Mansoor N, Vinknes KJ, Veierod MB, Retterstol K. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: A meta-analysis of randomised controlled trials. Br J Nutr. 2016;115:466–479. https://doi.org/10.1017/S0007114515004699
- Wood TR, Hansen R, Sigurethsson AF, Johannsson GF. The cardiovascular risk reduction benefits of a low-carbohydrate diet outweigh the potential increase in LDL-cholesterol. Br J Nutr. 2016;115:1126–1128. https://doi.org/10.1017/S0007114515005450
- Noakes TD. Low-carbohydrate and high-fat intake can manage obesity and associated conditions: Occasional survey. S Afr Med J. 2013;103:826–830. https://doi.org/10.7196/SAMJ.7302
- Feinman RD, Vernon MC, Westman EC. Low carbohydrate diets in family practice: What can we learn from an internet-based support group. Nutr J. 2006;5:26. https://doi.org/10.1186/1475-2891-5-26
- Paleo and low carb, 52 countries strong [homepage on the Internet]. 2013 [cited 2017 Aug 20]. Available from: http://www.awlr.org/results-2013.html
- Phelan S, Wyatt H, Nassery S, et al. Three-year weight change in successful weight losers who lost weight on a low-carbohydrate diet. Obesity (Silver Spring, MD). 2007;15:2470–2477. https://doi.org/10.1038/oby.2007.293
- Atkins RD. Dr. Atkins’ new diet revolution. New York: HarperCollins; 2002.
- Nielsen JV, Joensson E. Low-carbohydrate diet in type 2 diabetes. Stable improvement of bodyweight and glycemic control during 22 months follow-up. Nutr Metab (Lond). 2006;3:22. https://doi.org/10.1186/1743-7075-3-22
- Cerhan JR, Moore SC, Jacobs EJ, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89:335–345. https://doi.org/10.1016/j.mayocp.2013.11.011
- McSweeney JC, Rosenfeld AG, Abel WM, et al. Preventing and experiencing ischemic heart disease as a woman: State of the science: A statement for healthcare professionals from the American Heart Association. Circulation. 2016;133:1302–1331. https://doi.org/10.1161/CIR.0000000000000381
- Schöttker B, Rathmann W, Herder C, et al. HbA(1c) levels in non-diabetic older adults – No J-shaped associations with primary cardiovascular events, cardiovascular and all-cause mortality after adjustment for confounders in a meta-analysis of individual participant data from six cohort studies. BMC Med. 2016;14:26. https://doi.org/10.1186/s12916-016-0570-1
- Zhong GC, Ye MX, Cheng JH, Zhao Y, Gong JP. HbA1c and risks of all-cause and cause-specific death in subjects without known diabetes: A dose-response meta-analysis of prospective cohort studies. Sci Rep. 2016;6:24071. https://doi.org/10.1038/srep24071
- Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–811. https://doi.org/10.1056/NEJMoa0908359
- Pai JK, Cahill LE, Hu FB, Rexrode KM, Manson JE, Rimm EB. Hemoglobin A1c Is associated with increased risk of incident coronary heart disease among apparently healthy, nondiabetic men and women. J Am Heart Assoc. 2013;2:e000077. https://doi.org/10.1161/JAHA.112.000077
- Gaziano JM, Hennekens CH, O’Donnell CJ, Breslow JL, Buring JE. Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation. 1997;96:2520–2525. https://doi.org/10.1161/01.CIR.96.8.2520
- McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139:802–809. https://doi.org/10.7326/0003-4819-139-10-200311180-00007
- Da Luz PL, Favarato D, Junior J, Lemos P, Chagas ACP. High ratio of triglycerides to HDL-cholesterol predicts extensive coronary disease. Clinics. 2008;63:427–432.
- Krauss RM, Siri PW. Metabolic abnormalities: Triglyceride and low-density lipoprotein. Endocrinol Metab Clin North Am. 2004;33:405–415. https://doi.org/10.1016/j.ecl.2004.03.016
- Siri-Tarino PW, Chiu S, Bergeron N, Krauss RM. Saturated fats versus polyunsaturated fats versus carbohydrates for cardiovascular disease prevention and treatment. Annu Rev Nutr. 2015;35:517–543. https://doi.org/10.1146/annurev-nutr-071714-034449
- Boizel R, Benhamou PY, Lardy B, Laporte F, Foulon T, Halimi S. Ratio of triglycerides to HDL cholesterol is an indicator of LDL particle size in patients with type 2 diabetes and normal HDL cholesterol levels. Diabetes Care. 2000;23:1679–1685. https://doi.org/10.2337/diacare.23.11.1679
- Li C, Ford ES, Meng YX, Mokdad AH, Reaven GM. Does the association of the triglyceride to high-density lipoprotein cholesterol ratio with fasting serum insulin differ by race/ethnicity? Cardiovasc Diabetol. 2008;7:4. https://doi.org/10.1186/1475-2840-7-4
- Despres JP, Lamarche B, Mauriege P, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334:952–957. https://doi.org/10.1056/NEJM199604113341504
- Howard BV, Van Horn L, Hsia J, et al. Low-fat dietary pattern and risk of cardiovascular disease: The women’s health initiative randomized controlled dietary modification trial. JAMA. 2006;295:655–666. https://doi.org/10.1001/jama.295.6.655
- Multiple Risk Factor Intervention Trial. Risk factor changes and mortality results. Multiple Risk Factor Intervention Trial Research Group. JAMA. 1982;248:1465–1477. https://doi.org/10.1001/jama.1982.03330120023025
- Volek JS, Fernandez ML, Feinman RD, Phinney SD. Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Prog Lipid Res. 2008;47:307–318. https://doi.org/10.1016/j.plipres.2008.02.003
- Ravnskov U, Diamond DM, Hama R, et al. Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: A systematic review. BMJ Open. 2016;6:e010401. https://doi.org/10.1136/bmjopen-2015-010401
- Volaklis KA, Halle M, Meisinger C. Muscular strength as a strong predictor of mortality: A narrative review. Eur J Intern Med. 2015;26:303–310. https://doi.org/10.1016/j.ejim.2015.04.013
- Stanaway FF, Gnjidic D, Blyth FM, et al. How fast does the grim reaper walk? Receiver operating characteristics curve analysis in healthy men aged 70 and over. BMJ. 2011;343:d7679. https://doi.org/10.1136/bmj.d7679
- Ruiz JR, Sui X, Lobelo F, et al. Association between muscular strength and mortality in men: Prospective cohort study. BMJ. 2008;337:92–95. https://doi.org/10.1136/bmj.a439
- Brito LB, Ricardo DR, Araujo DS, Ramos PS, Myers J, Araujo CG. Ability to sit and rise from the floor as a predictor of all-cause mortality. Eur J Prev Cardiol. 2014;21:892–898. https://doi.org/10.1177/2047487312471759
- Schneiderhan J, Clauw D, Schwenk TL. Primary care of patients with chronic pain. JAMA. 2017;317:2367–2368. https://doi.org/10.1001/jama.2017.5787
- Treating depression and anxiety in primary care. Prim Care Companion J Clin Psychiatry. 2008;10:145–152. https://doi.org/10.4088/PCC.v10n0209
- Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: A patient-level meta-analysis. JAMA. 2010;303:47–53. https://doi.org/10.1001/jama.2009.1943
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252–260. https://doi.org/10.1056/NEJMsa065779
- Goyal M, Singh S, Sibinga EMS, et al. Meditation programs for psychological stress and well-being: A systematic review and meta-analysis. JAMA Intern Med. 2014;174:357–368. https://doi.org/10.1001/jamainternmed.2013.13018
- Jacka FN, O’Neil A, Opie R, et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017;15:23. https://doi.org/10.1186/s12916-017-0791-y
- Kok RM, Reynolds CF III. Management of depression in older adults: A review. JAMA. 2017;317:2114–2122. https://doi.org/10.1001/jama.2017.5706
- Bostock ECS, Kirkby KC, Taylor BVM. The current status of the ketogenic diet in psychiatry. Front Psychiatry. 2017;8:43. https://doi.org/10.3389/fpsyt.2017.00043
- Nickols-Richardson SM, Coleman MD, Volpe JJ, Hosig KW. Perceived hunger is lower and weight loss is greater in overweight premenopausal women consuming a low-carbohydrate/high-protein vs high-carbohydrate/low-fat diet. J Am Diet Assoc. 2005;105:1433–1437. https://doi.org/10.1016/j.jada.2005.06.025
- Hu T, Yao L, Reynolds K, et al. The effects of a low-carbohydrate diet on appetite: A randomized controlled trial. Nutr Metab Cardiovasc Dis. 2016;26:476–488. https://doi.org/10.1016/j.numecd.2015.11.011
- Merra G, Miranda R, Barrucco S, et al. Very-low-calorie ketogenic diet with aminoacid supplement versus very low restricted-calorie diet for preserving muscle mass during weight loss: A pilot double-blind study. Eur Rev Med Pharmacol Sci. 2016;20:2613–2621.
- Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med. 2005;142:403–411. https://doi.org/10.7326/0003-4819-142-6-200503150-00006
- Van Cappellen P, Rice EL, Catalino LI, Fredrickson BL. Positive affective processes underlie positive health behaviour change. Psychol Health. 2017:1–21. https://doi.org/10.1080/08870446.2017.1320798
- Di Lorenzo C, Coppola G, Sirianni G, et al. Migraine improvement during short lasting ketogenesis: A proof-of-concept study. Eur J Neurol. 2015;22:170–177. https://doi.org/10.1111/ene.12550
- Austin GL, Dalton CB, Hu Y, et al. A very low-carbohydrate diet improves symptoms and quality of life in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7:706–708.e1. https://doi.org/10.1016/j.cgh.2009.02.023
- Austin GL, Thiny MT, Westman EC, Yancy WS, Shaheen NJ. A very low-carbohydrate diet improves gastroesophageal reflux and its symptoms. Dig Dis Sci. 2006;51:1307–1312. https://doi.org/10.1007/s10620-005-9027-7
- Mavropoulos JC, Yancy WS, Hepburn J, Westman EC. The effects of a low-carbohydrate, ketogenic diet on the polycystic ovary syndrome: A pilot study. Nutr Metab. 2005;2:35. https://doi.org/10.1186/1743-7075-2-35
- Tendler D, Lin S, Yancy WS, et al. The effect of a low-carbohydrate, ketogenic diet on nonalcoholic fatty liver disease: A pilot study. Dig Dis Sci. 2007;52:589–593. https://doi.org/10.1007/s10620-006-9433-5
- Masino SA, Ruskin DN. Ketogenic diets and pain. J Child Neurol. 2013;28:993–1001. https://doi.org/10.1177/0883073813487595
- Dieleman JL, Baral R, Birger M, et al. US spending on personal health care and public health, 1996–2013. JAMA. 2016;316:2627–2646. https://doi.org/10.1001/jama.2016.16885
- Jia H, Lubetkin EI. The impact of obesity on health-related quality-of-life in the general adult US population. J Public Health. 2005;27:156–164. https://doi.org/10.1093/pubmed/fdi025
- Gates DM, Succop P, Brehm BJ, Gillespie GL, Sommers BD. Obesity and presenteeism: The impact of body mass index on workplace productivity. J Occup Environ Med. 2008;50:39–45. https://doi.org/10.1097/JOM.0b013e31815d8
SOURCE: insulinresistance











